So, understanding molecular bonding, properties, and structures starts with understanding ground state electron configuration.
Thus, the ground state electron configuration sheds light on many different atomic properties, from the electrons in an atom to the different orbitals and hybridization. Therefore, the understanding of electron configuration is fundamental to comprehending the periodic table.
Moreover, Niels Bohr proposed in 1913 that electrons could orbit an atom at a specific distance without collapsing into it, with each orbit distance having its own energy level.
Read Also: Gravitational force formula- All you need to know
Additionally, he proposed that the angular momentum of each orbital, M, was equal to n times Plank’s constant, h, divided by 2. Thus, this yields the equation M = nħ, where n = 1,2,3,4 and ħ= h/2π. This model proposed that the Bohr atom has circular orbits surrounding the nucleus. The ground state electron configuration of certain atoms is important to learn.
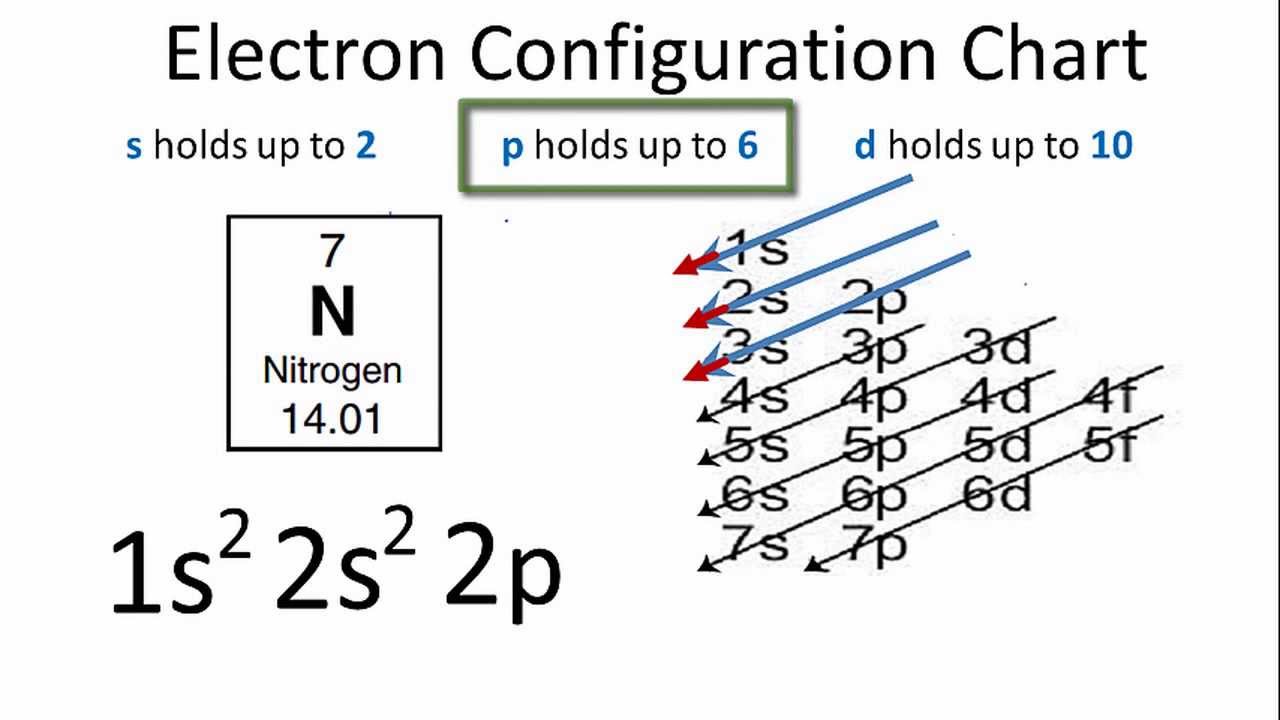
The Ground State Electron Configuration Of Nitrogen Atom Can Be Represented By
Therefore, we can only represent the electronic configuration of the nitrogen atom in its ground state by 1, 4.
Moreover, we align the spins of all unpaired electrons in the same direction.
So, we fill the electrons in orbitals in the ground state electronic configuration of nitrogen.
Thus, we do this according to the Aufbau principle, Hund’s rule of maximum spin multiplicity, and Pauli’s principle.
As a result, the first three unpaired electrons filled the three orbitals.
The Ground State Electron Configuration Of Carbon
So, the electron configuration is the arrangement of electrons in an atom’s atomic orbitals.
Thus, we can use a periodic table to determine electron configurations.
Also, Carbon is the sixth element in the periodic table, with a total of six electrons.
As a result, the atomic number Z equals 6.
So, Carbon’s electron configuration in its ground state is 1s2 2s2 2p2. Carbon has an excited state electron configuration of 1s2 2s1 2p3.

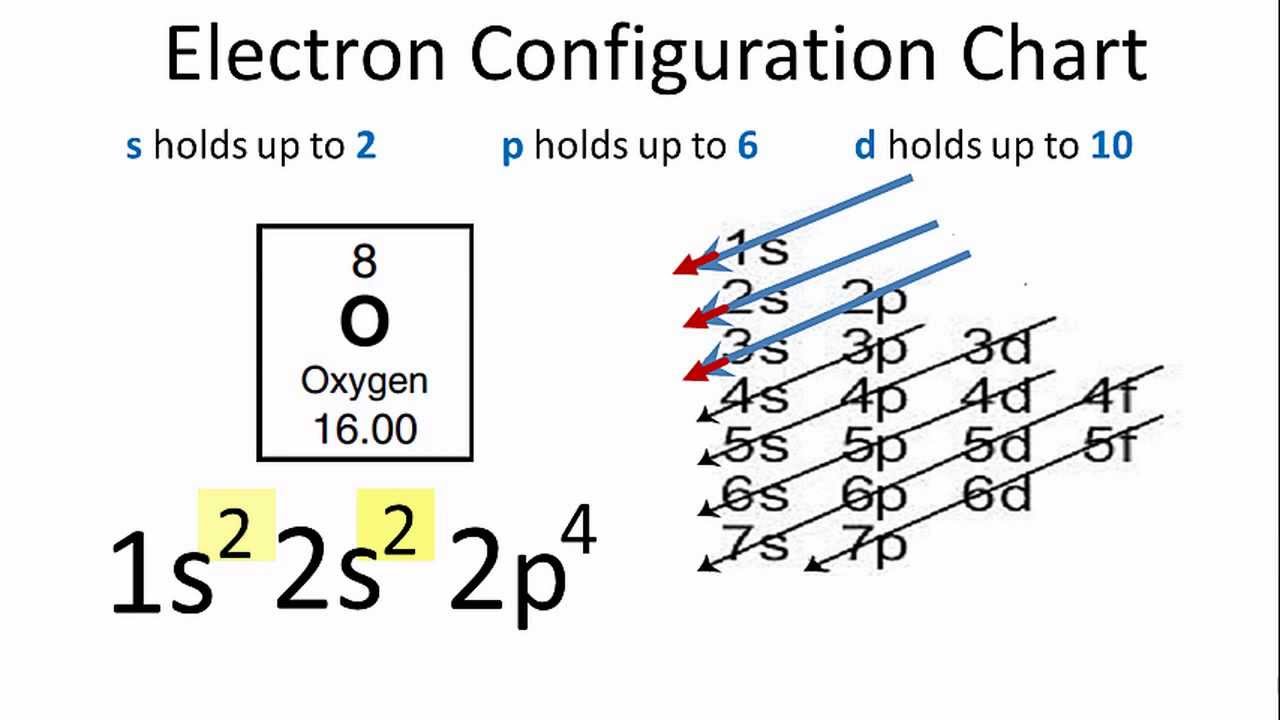
Ground State Electron Configuration Of Oxygen
The valence electron has (typically) moved from its ground state orbital (i.e. lowest available energy) to a higher energy orbital in an excited state.
So, now let us define an excited state for this element.
Moreover, it goes as follows.
Any electron configuration in which the last electron (the valence electron) is in a higher energy orbital.
Moreover, for example, the electron configuration of oxygen’s ground state (electrons in the energetically lowest available orbital) is 1s2 2s2 2p4.
However, the electron could occupy an infinite number of orbitals if the element became excited.
In most texts, however, the next available example will be used.
So, in the case of oxygen, it might look something like this: 2s2 2p3 1s2 2s2 3s1. Thus, here, the valence electron is now in an excited (not ground) state in the 3s orbital.
Ground State Electron Configuration Of Phosphorous
The shell structure of phosphorus atoms is 2.8.5 and they have 15 electrons.
So, the term symbol for ground state gaseous neutral phosphorus is 4S3/2.
Also, the ground state electron configuration is [Ne] 3s2 3p3.

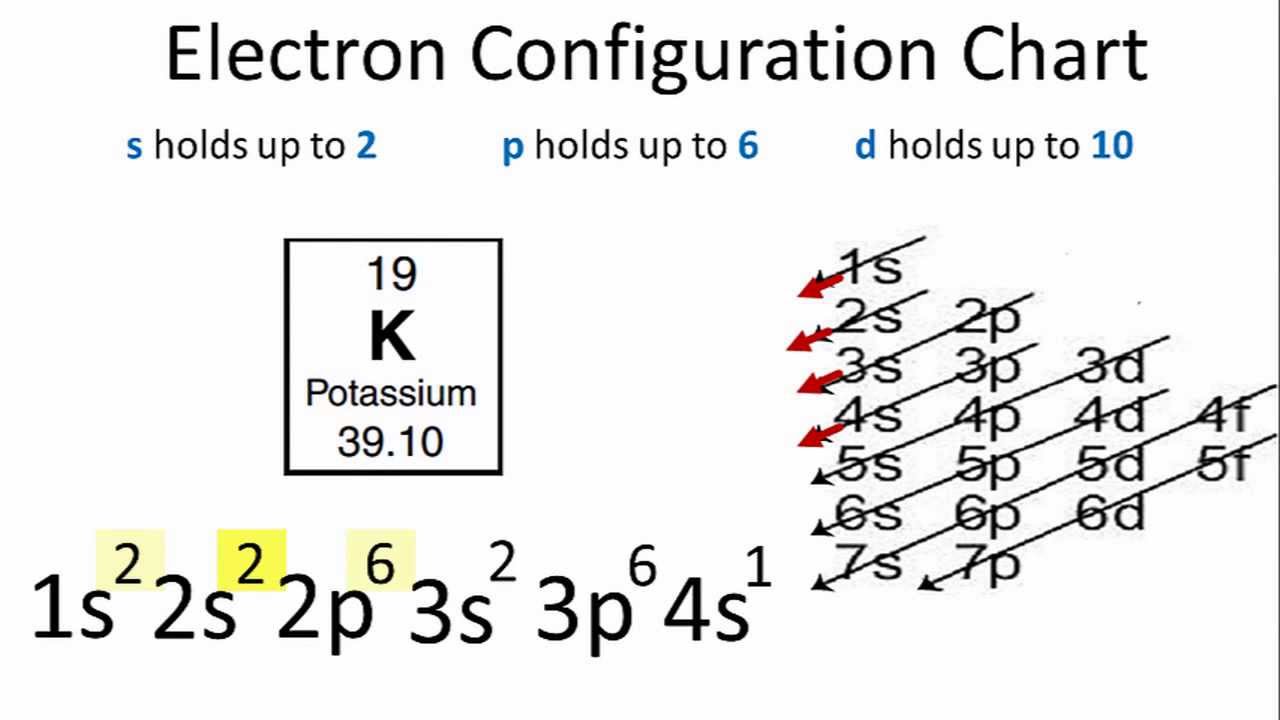
Ground State Electron Configuration Of Potassium
So, the atom of potassium (K) has 19 electrons. Potassium (K) has a full electron configuration of 1s2 2s2 2p6 3s2 3p6 4s1.
Also, the abbreviated form [Ar]4s1 denotes Argon’s (Ar) electron configuration plus one electron in the 4s orbital.
Moreover, Argon has a total of 18 electrons. So, one more electron configuration completes the picture for Potassium’s 19 electrons.
Ground State Electron Configuration Of Chromium
The half-filled 3d subshell with 5 electrons is more stable than the full subshell with 4 electrons. So, for more stability, 1,4s electrons jump into the 3d subshell.
Therefore, as a result, the ground state electron configuration of Chromium is [Ar] 3d5 4s1.

Ground State Electron Configuration Of CU
Cu has an atomic number of 29.
So, Cu 29 =1s2 2s2 2p6 3s2 3p6 3d10 4s1 =1s2 2s2 2p6 3s2 3p6 3d10 4s1 =1s2 2s2 2p6 3s2 3p6 3d10 4s1 =1s2 2s2
Because fully filled orbitals are more stable, an electron from the 4s orbital moves to the 3d orbital.
And, Ne10 =1s2 2s2 2p6 is the electronic configuration of neon.
We can also represent Cu’s configuration by using inert gas in the following way:
So, Cu29 =[Ne] 3s2 3p6 3d10 4s1.

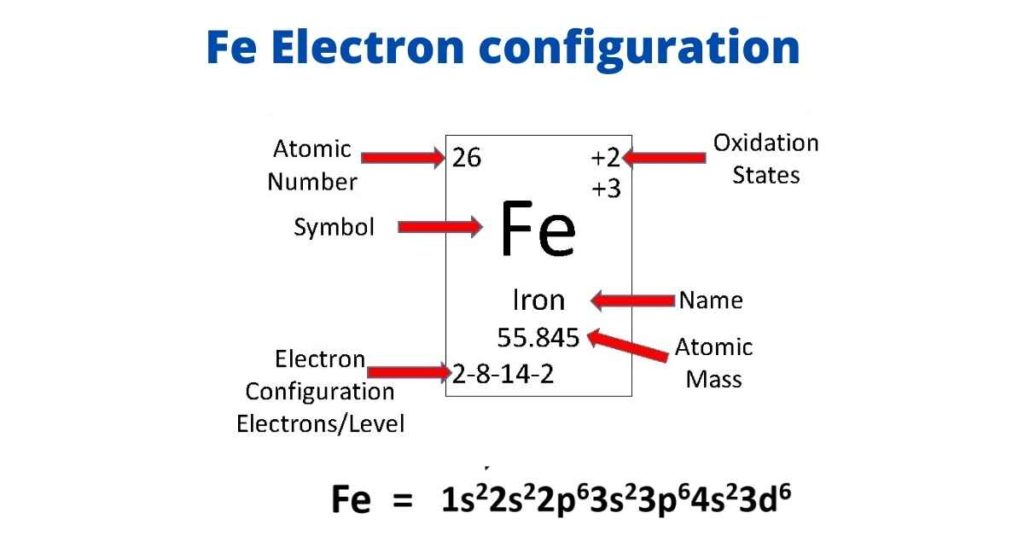
Ground State Electron Configuration Of Fe
Iron’s electron configuration in its ground state is 1s2 2s2 2p6 3s2 3p6 3d6 4s2. The last shell of iron has two electrons, and the d-orbital has a total of six electrons, as shown in this electron configuration. Iron has eight valence electrons as a result of this.
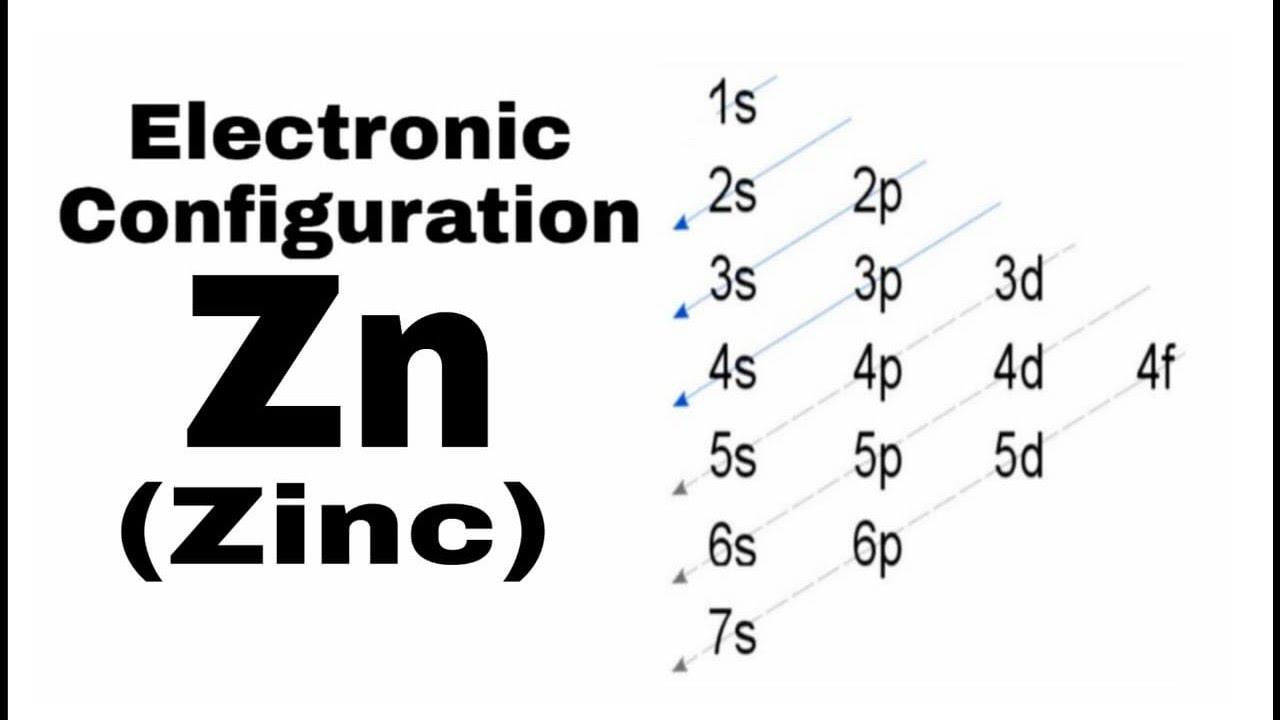
Ground State Electron Configuration Of Zn
Thus, the ground state electron configuration of gaseous neutral zinc in its ground state is [Ar]. 3d10. 4s2, with 1S0 as the term symbol.
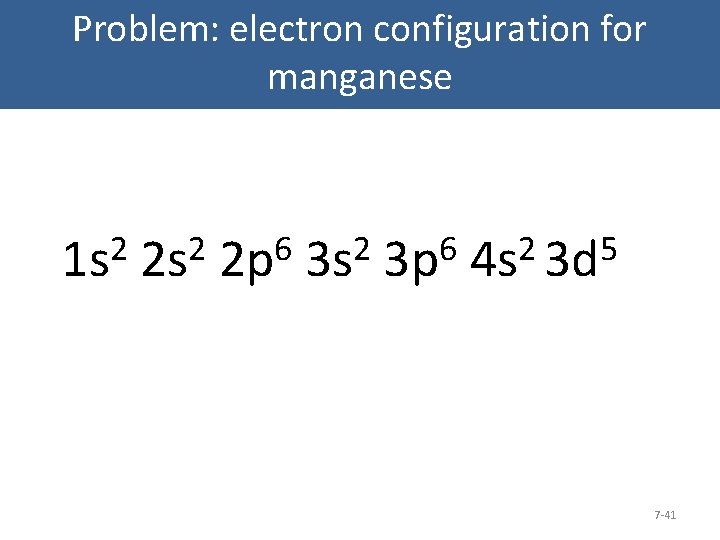
Ground State Electron Configuration Of Mn
Manganese’s (Mn) period number is 4. Also, its atomic number is 25.
Thus, as a result, its electronic configuration is either 1s2 2s2 2p6 3s2 3p6 4s2 4d5 or [Ar] 4s2 3d5.
Ground State Electron Configuration Of Iodine
The term symbol for ground-state gaseous neutral iodine is 2P3/2. And the ground state electron configuration is [Kr] 4d10 5s2 5p5.
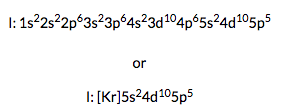
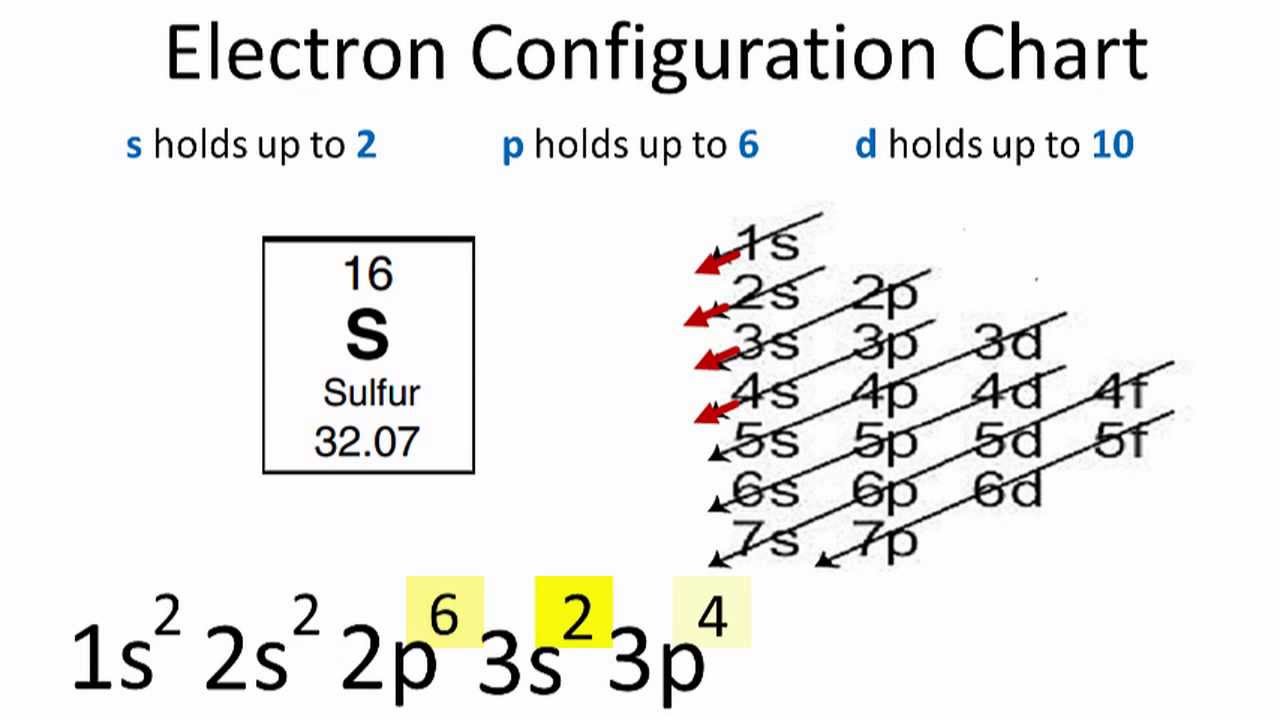
Ground State Electron Configuration Of Sulphur
The first two electrons in Sulfur’s electron configuration will be in the 1s orbital. Because the 1s orbital can only hold two electrons, we assign sulphur’s next two electrons to the 2s orbital.
So, The following six electrons will be in the 2p orbital.
The p orbital can hold up to six electrons at a time.
We will place six electrons in the 2p orbital, followed by two electrons in the 3s orbital.
Then, we will move to the 3p to place the remaining four electrons because the 3s is now full. As a result, the electron configuration of sulphur will be 1s2 2s2 2p6 3s2 3p4.
What Is The Ground State Electron Configuration Of B
The first two electrons in the electron configuration for Boron will be in the 1s orbital.
Because the 1s orbital can only hold two electrons, we place B’s next two electrons in the 2s orbital.
We will place the final electron in the 2p orbital.
As a result, the electron configuration of the B electron will be 1s22s22p1.

What Is The Ground State Electron Configuration Of Calcium
In the electron configuration for Calcium, the first two electrons would be in the 1s orbital.
Because the 1s orbital can only hold two electrons, we will place Calcium’s next two electrons in the 2s orbital.
So, the following six electrons will be in the 2p orbital.
The p orbital can hold up to six electrons at a time.
We will place six electrons in the 2p orbital, followed by two electrons in the 3s orbital.
So, we will move to the 3p to place the next six electrons because the 3s are now full.
Then, we will now place the remaining two electrons in the 4s orbital.
As a result, the electron configuration of Calcium will be 1s2 2s2 2p6 3s2 3p6 4s2.

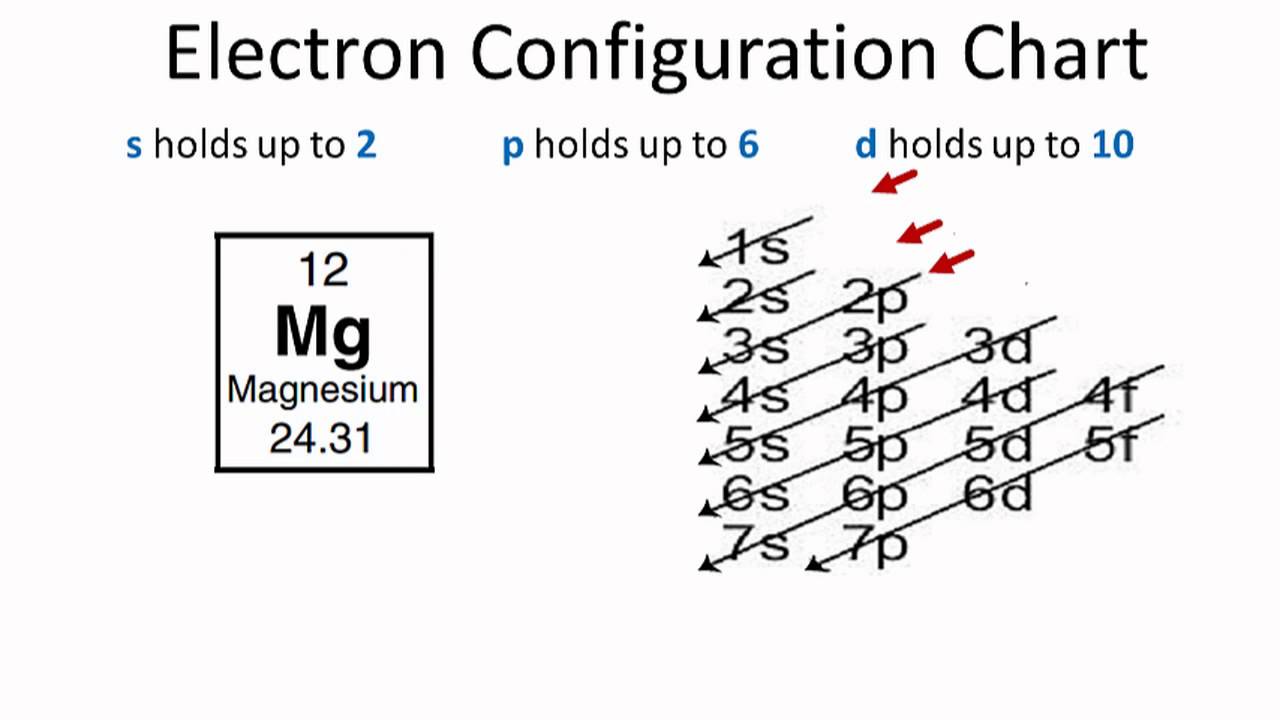
Ground State Electron Configuration Of Magnesium
The first two electrons in Magnesium’s electron configuration will be in the 1s orbital.
Because the 1s orbital can only hold two electrons, we will place the next two electrons for magnesium in the 2s orbital.
Again, we will see that the nexus’s six electrons will be in the 2p orbital.
The p orbital can hold up to six electrons at a time.
We will place the six electrons in the 2p orbital, and we will place the remaining two in the 3s orbital.
As a result, the electron configuration of Magnesium will be 1s2 2s2 2p6 3s2.
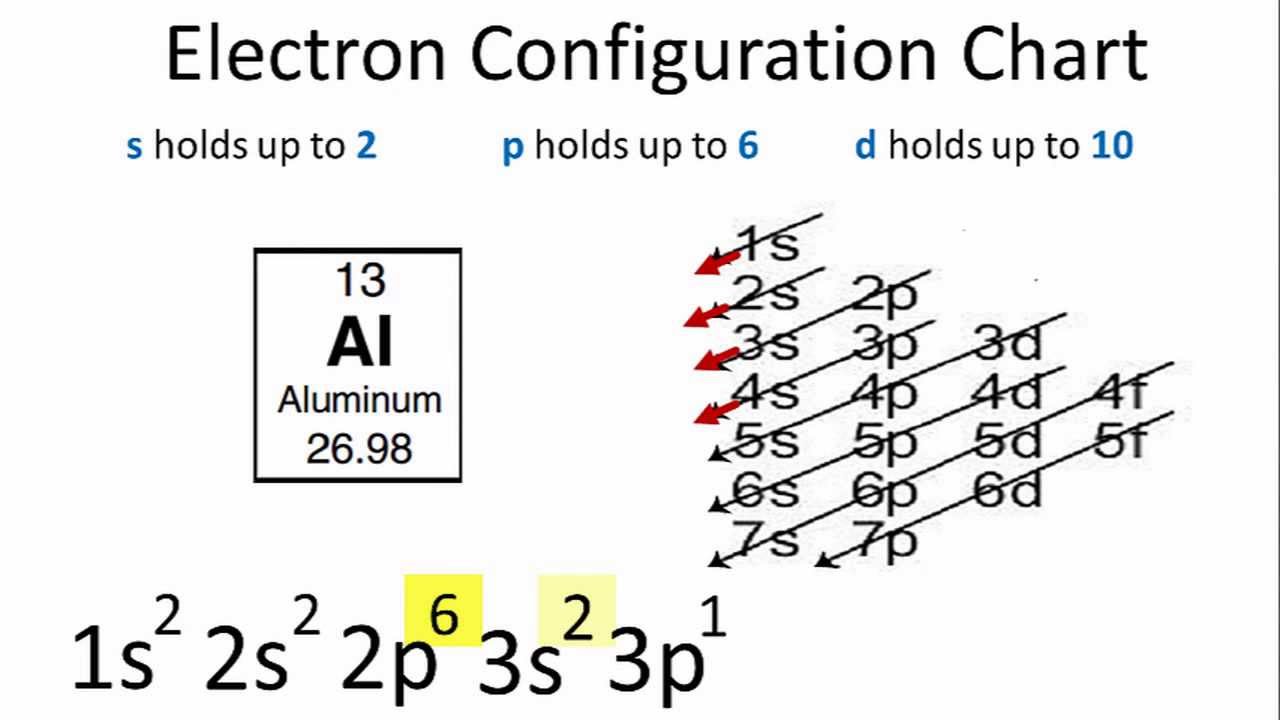
Ground State Electron Configuration Of Aluminium
The first two electrons in the electron configuration for Aluminium will be in the 1s orbital.
Because the 1s orbital can only hold two electrons, we will place the next two electrons for aluminum in the 2s orbital.
Again, we will see that the nexus’s six electrons will be in the 2p orbital.
The p orbital can hold up to six electrons at a time.
We will place the six electrons in the 2p orbital, followed by two electrons in the 3s orbital.
We will move the remaining electron from the 3s to the 3p because the 3s is now full.
As a result, the electron configuration of Aluminum will be 1s2 2s2 2p6 3s2 3p1.
Electron Configuration Of Mo
The electron configuration of molybdenum in its ground state is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d5 5s1. The last shell of molybdenum has an electron, and the d-orbital has a total of five electrons in this electron configuration. As a result, molybdenum has six valence electrons.

Electron Configuration Of Ag
Silver’s electron configuration is determined by its location in the fifth row of the periodic table, in the 11th column of the periodic table, or in the 9th column of the transition metal or d block. As a result, silver’s electron configuration must be 4s9 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d9
By replacing the 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 with the noble gas, this notation can be written in core or noble gas notation. [Kr].
[Kr] 5s2 4d9
They will actually transfer a s electron to complete the d orbital in some transition metals, resulting in silver.
[Kr] 5s1 4d10
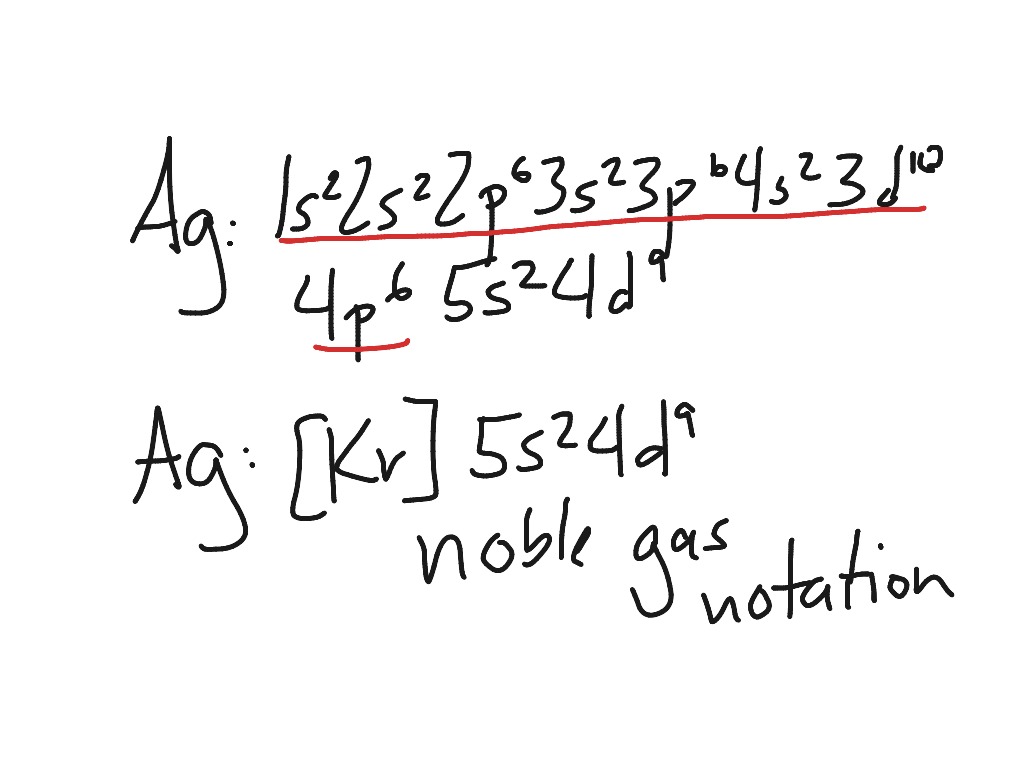
Electronic Configuration Of Argon
The first two electrons in Argon’s electron configuration will be in the 1s orbital. Because the 1s orbital can only hold two electrons, Argon’s next two electrons are assigned to the 2s orbital.
Again, the six electrons after that will be in the 2p orbital.
The p orbital can hold up to six electrons at a time.
Then, six electrons will be placed in the 2p orbital, followed by two electrons in the 3s orbital.
So, we will move to the 3p to place the remaining six electrons because the 3s is now full.
As a result, the electron configuration of Argon will be 1s2 2s2 2p6 3s2 3p6.

Electronic Configuration Of Neon
Neon is the tenth element in the periodic table, with a total of ten electrons.
So, the first two electrons in the electron configuration for neon will be in the 1s orbital.
Because the 1s orbital can only hold two electrons, Ne’s next two electrons are placed in the 2s orbital.
Also, the final six electrons will be assigned to the 2p orbital.
As a result, the electron configuration of the Ne atom will be 1s2 2s2 2p6.
Because there are eight electrons in the second energy level (2s2 2p6) Neon is an octet with a complete outer shell. As a result, it has been identified as a Nobel Gas.

Ground State Electron Configuration Meaning
Electrons, like us, have their own home in the world of subatomic particles. The electron configuration, which describes the electron arrangement within an atom, determines where they go.
There is a ground state and an excited state for electrons.
Again, we have already discussed the ground state electron configuration in this article.
When we speak of the ground state electron configuration, we’re referring to an atom’s electron configuration at the lowest possible energy level.
The natural state of a system in the universe is to use the least amount of energy possible.
So we will arrange an atom’s electrons at the ground state in the lowest energy state possible for that atom.
What Is Electron Configuration Formula
The maximum number of electrons that can be accommodated in a shell is determined by the principal quantum number (n).
Moreover, the shell number is represented by the formula 2n^2, where ‘n’ is the number of shells.
Thus, below is a table listing the shells, n values, and the total number of electrons that can be accommodated.
| Shell and n value | In the shell, what is the maximum number of electrons |
| K shell, n = 1 | 2 * 1^2 = 2 |
| L shell, n = 2 | 2 * 2^2 = 8 |
| M shell, n = 3 | 2 * 3^2 = 18 |
| N shell, n = 4 | 2 * 4^2 = 32 |
Ground state electron configuration FAQs
1. What Are Electron Configurations In The Ground State?
Let’s go over everything again. The Ground State Electron Configuration is an arrangement of lower energy electrons around the nucleus of an atom. Electrons occupying various energy levels’ orbitals naturally gravitate towards the lowest energy state or ground state.
2. What Is the Importance of Electron Configurations?
The Crucial Point Is That We Recognize That Understanding Electron Configurations Aids Us In Determining The Valence Electrons On An Atom.
Thus, this is significant because valence electrons play a role in each atom’s unique chemistry.
So, when describing an electron configuration in terms of orbital diagrams, this is critical.
3. Magnesium has how many orbitals?
You already know that the atomic number of Magnesium indicates the number of electrons in the element.
Thus, in a Magnesium Atom, this means there are 12 electrons. There are two electrons in Shell One, eight in Shell Two, and two more in Shell Three, as shown in the diagram below.

4. What does 1s 2s 2p 3s 3p stand for?
We represent the electron orbital energy levels by the letters 1s 2s 2p 3s 3p.
5. The electron configuration of which element is 1s 2 2s 2 2p 6 3s 2 3p 4?
This element is sulphur, according to the periodic table.
6. What are the three principles that govern electron configurations?
We all must follow the Aufbau Principle, the Pauli-Exclusion Principle, and Hund’s Rule when assigning electrons to orbitals.
7. What are the electron configuration rules’ exceptions?
Only chromium and copper are exceptions to electron configuration.
Because a fully or partially filled d sub-level is more stable than a partially filled d sub-level in these circumstances, an electron from the 4s orbital becomes excited and rises to a 3d orbital.
8. What is the electron configuration of the element Kr in its ground state?
Krypton has 36 electrons in total. So, the element’s electronic configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6.
9. What is the electron configuration of xenon in its ground state?
Xenon’s full electron configuration is as follows: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6.
10. Give an example of an electron configuration.
The location of electrons around the nucleus of an atom is described by electron configurations.
Lithium, for example, has two electrons in the 1s subshell and one electron in the 2s subshell, as indicated by its electron configuration of 1s2 2s1.